COMBIPATCH® Dosing Regimens
There are two hormone therapy regimens that use COMBIPATCH: The Continuous Combined regimen and Continuous Sequential regimen.
- The Continuous Combined regimen with COMBIPATCH provides a consistent level of estrogen and progestin throughout the month. Women prescribed continuous combined treatment may experience spotting, particularly in the first six months, but it generally decreases over time
- More about the Continuous Combined regimen
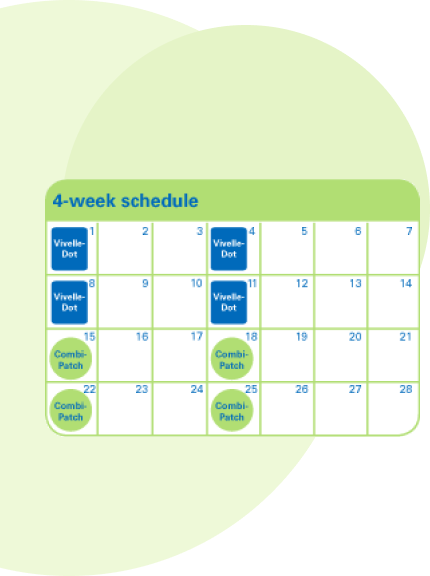
- The Continuous Sequential regimen with COMBIPATCH and Minivelle® (estradiol transdermal system) provides a consistent level of estrogen throughout the month, but progestin during only half of the month. Women prescribed the Continuous Sequential regimen often experience a predictable monthly bleed.
- In this regimen, Minivelle is applied for the first two weeks of the month, followed by COMBIPATCH during weeks 3 and 4
- You may experience regular monthly bleeding